
Ron Waksman, MD
Principal Investigator, LRP Study
MedStar Washington Hospital Center
The landmark Lipid-Rich Plaque (LRP) study was designed to demonstrate that intravascular NIRS imaging can identify patients and plaques at risk for subsequent non-culprit major adverse cardiovascular events (NC-MACE) during a 24-month period. The results were presented by Dr. Ron Waksman and published in The Lancet on September 27, 2019.
The study successfully met both its co-primary endpoints as well as its key secondary endpoints, providing clinical evidence that intravascular NIRS imaging can accurately identify both vulnerable patients and vulnerable plaques that are at significantly higher risk for subsequent NC-MACE.
Learn moreStudy Co-Primary Endpoints
Association between maxLCBI4mm in all imaged arteries and future patient-level nonculprit MACE*
Association between maxLCBI4mm in a segment and incidence of future NC-MACE* in same segment
Study Secondary Endpoints
A threshold of maxLCBI4mm>400 will identify patients and plaques at increased risk of a NC-MACE*
*Non-culprit MACE (independently adjudicated by CEC blinded to NIRS)
Firmly established that LCBI correlates to MACE risk and should be managed.
Firmly established that LCBI correlates to MACE risk and should be managed
89%
increased risk
for patients with overall maxLCBI4mm>400
18%
increased risk
for each 100 unit increase in overall maxLCBI4mm
322%
increased risk
for each 30mm Ware segment with maxLCBI4mm>400
45%
increased risk
for each 100 unit increase in maxLCBI4mm for each Ware segment
Key findings as published in The Lancet on September 27, 2019
The following example represents a typical case study of a patient enrolled in the LRP study and the subsequent observations of that patient.
A 44-year-old male with a past medical history of coronary artery disease, diabetes mellitus, hypertension and hyperlipidemia, presented with chest and right leg pain was imaged and scanned on April 30, 2014. The angiogram showed some non-obstructive plaque; the chemogram indicated that maxLCBI4mm was greater than 400, an indication of risk for subsequent NC-MACE. Twenty-two months later, the patient presented with unstable angina, which required percutaneous coronary intervention (PCI). New NIRS imaging showed that the new culprit lesion was located within the Ware segment previously identified on the chemogram.
LRP Case Study Example
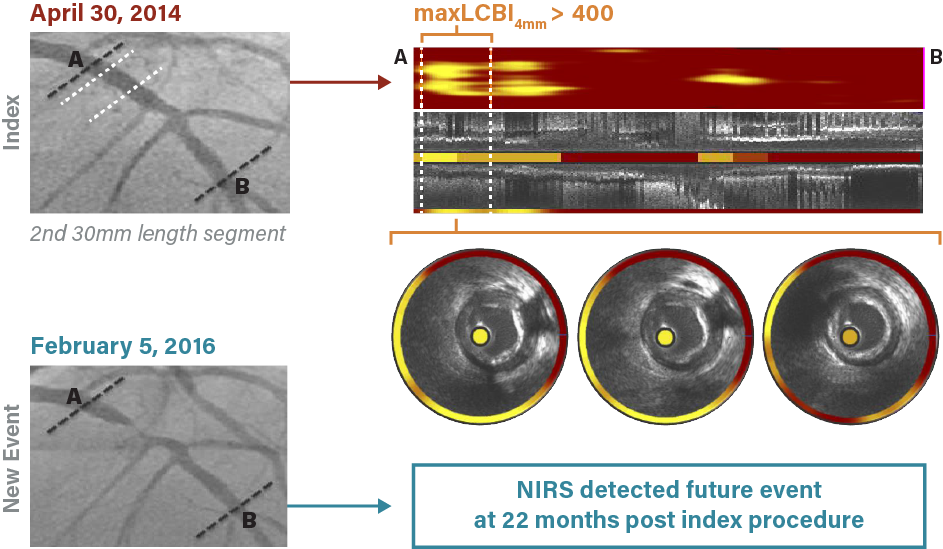
As presented by Dr. Ron Waksman and published in The Lancet on September 27, 2019.
The Makoto™ Intravascular Imaging System is an FDA-cleared imaging system indicated for the detection of plaque and patients at higher risk of MACE.
To request more information on how to obtain the Makoto™ system or its accompanying Dualpro™ IVUS+NIRS Catheter, or to see a demo, CLICK HERE
© 2024 Infraredx™, Inc. All Rights Reserved Powered by Bloom Creative